Gilead Sciences, Inc. announced it is issuing a voluntary recall of one lot of Veklury (remdesivir) for Injection 100mg/vial, to the consumer level. Gilead received a customer complaint and confirmed the presence of a glass particle in the vial during the company’s investigation.
Risk Statement: The administration of an injectable product that contains glass particles may result in local irritation or swelling in response to the foreign material. The glass particulate can potentially travel, through the blood vessels, to various organs and block blood vessels in the heart, lungs or brain which can cause stroke and even lead to death. To date, Gilead has not received any reports of adverse events related to this recall.
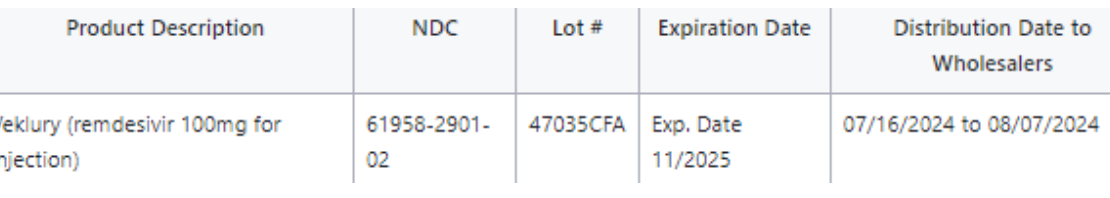
Consumers and healthcare providers with questions regarding this recall can contact Gilead Medical Information at 1-866-633-4474 Monday to Friday 5am – 6pm PST or through their website at www.askgileadmedical.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product. This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Source: Gilead Sciences, Inc.


