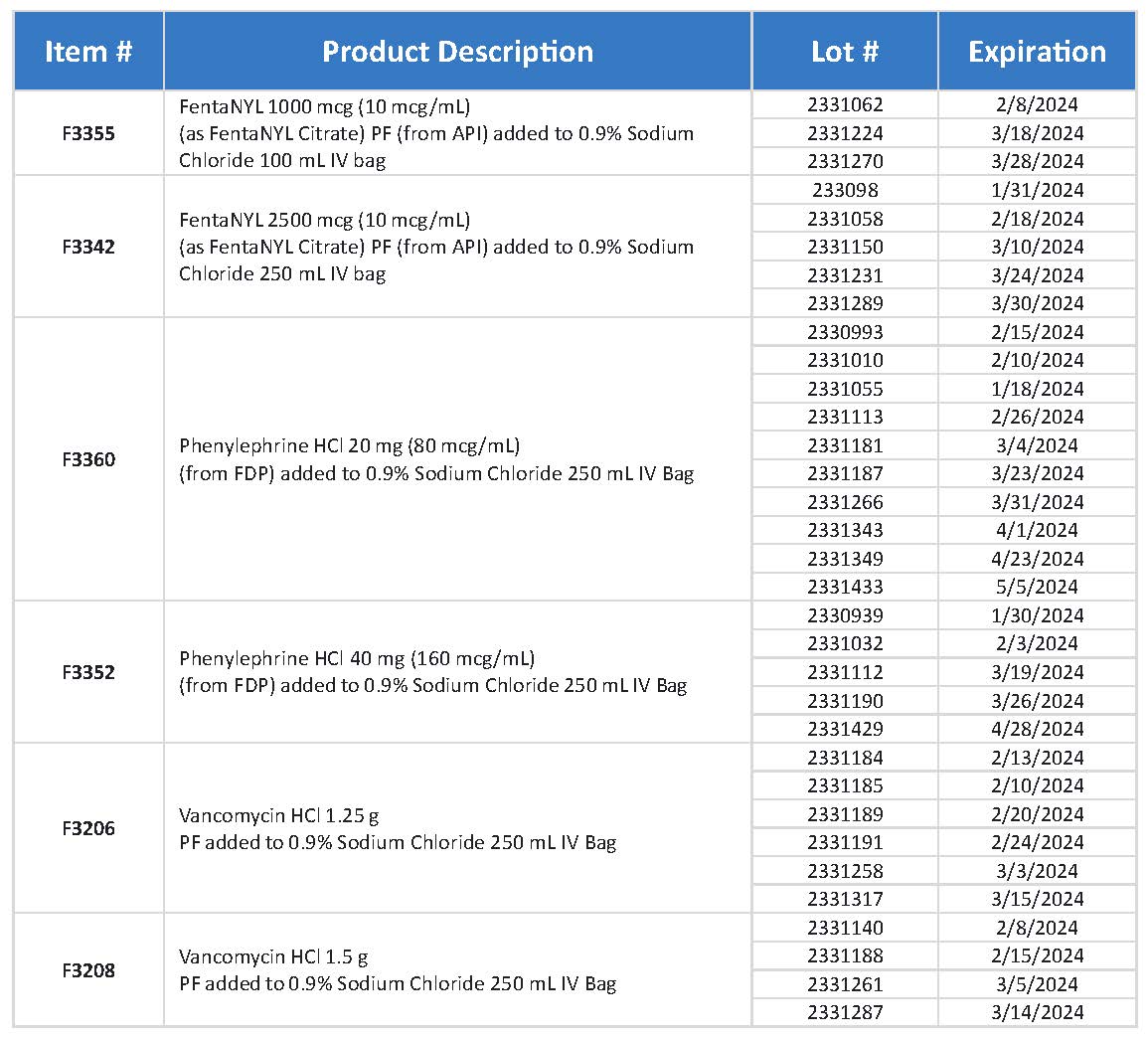
Leiters Health is voluntarily recalling 33 lots of products listed below to the user level. The recalled batches of vancomycin IV bags, phenylephrine IV bags, and fentanyl IV bags are being recalled due to the potential for superpotency because they may contain twice the labeled amount of drug.
The semi-automated IV bag filling equipment used to fill the recalled batches may not eject the IV bags properly when compressed air tanks become low or a leak was detected, causing the recalled IV bags to be dosed twice.
To date, Leiters Health has not received any reports of adverse events related to this recall.
Fentanyl is an analgesic packaged in an IV bag under codes F3355 and F3342. Phenylephrine is used for perioperative hypotension, hypotension during anesthesia, and shock and is packaged in an IV bag under codes F3360 and F3352.
Vancomycin is used for endocarditis and staphylococcal infections and is packaged in an IV bag under codes F3206 and F3208.
The products were distributed nationwide to hospitals for administration in the hospital. Leiters Health has notified its customers by a letter; sent via mail, requiring signature upon receipt, and an email to all affected customers. Leiters Health is arranging for a credit for all recalled products. Customers that have a product which is being recalled should cease using it and return it to Leiters Health.
Consumers with questions regarding this recall can contact Leiters Health by phone at 1-800-292-6772 or email at recall@leiters.com Monday through Friday between 8:00 AM MST and 5:30 PM MST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using these drug products. Customers will receive return shipping labels for phenylephrine and vancomycin returns via email from Leiters Health, to return their products to Leiters Health at 13796 Compark Blvd., Englewood, CO 80112. Customers will receive return shipping labels, along with a DEA Form 222, for fentanyl returns via mail from Leiters Health, to return their products to Leiters Health at 13796 Compark Blvd., Englewood, CO 80112.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
• Complete and submit the report Online
• Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then
complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178


