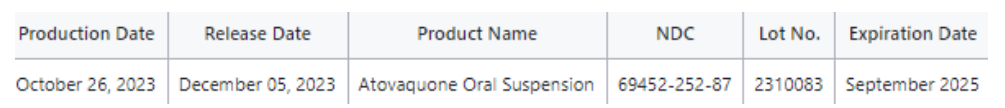
Bionpharma Inc. is voluntarily recalling (1) single Batch (2310083) of Atovaquone Oral Suspension, 750mg per mL to the consumer level. The product was manufactured by CoreRx, Inc. in Clearwater, FL and distributed by Bionpharma Inc. The product was found to be contaminated with Cohnella bacteria.
Risk Statement: In the population most at risk, immunocompromised population, there is a reasonable probability that microbial contamination of Atovaquone Oral Suspension can result in disseminated, life threatening infections such as inflammation of the heart and permanent damage to soft tissue. To date, Bionpharma has not received any reports of adverse events related to this recall.
Consumers with questions regarding this recall can contact Bionpharma by phone at (888) 235-2466 (Mon-Fri 9AM-5PM EST) or via email to drugsafety@bionpharma.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using the affected lot of the drug product.
Source: FDA
Note: No BCF members were affected by this recall.


