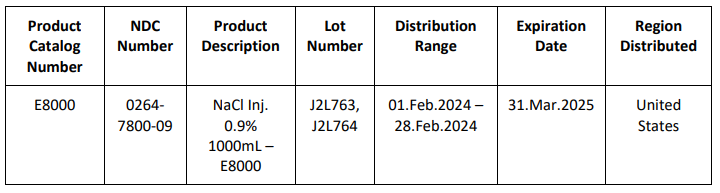
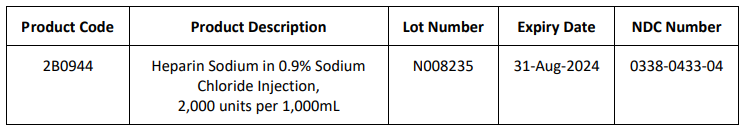
FDA Approves Niktimvo for the Treatment of Chronic Graft-Versus-Host Disease
Incyte and Syndax Pharmaceuticals announced the U.S. Food and Drug Administration (FDA) approved Niktimvo (axatilimab-csfr), a colony stimulating factor-1 receptor (CSF-1R)-blocking antibody, for the treatment of chronic graft-versus-host disease (GVHD) after failure of at least two prior lines of systemic therapy in adult and pediatric patients weighing at least 40kg (88.2lbs).
- Chronic GVHD is a serious condition that can occur after an allogeneic stem cell transplant (the transfer of stem cells from a donor) in which the donated cells initiate an immune response and attack the transplant recipient’s organs.
- Chronic GVHD is a leading cause of significant morbidity and mortality after an allogeneic stem cell transplant and is estimated to develop in approximately 42% of transplant recipients, affecting approximately 17,000 patients in the U.S. Of those patients who develop chronic GVHD, nearly 50% require at least three lines of treatment, emphasizing the need for additional effective treatment options.
Source: Incyte and Syndax Pharmaceuticals